Even in the most secure drug supply systems in the world, the distribution of pharmaceutical products can be seriously impacted by illegal activities such as drug theft, drug diverting, and even drug counterfeiting.
On this issue, the World Health Organization (WHO) estimates that more than 10 percent of the prescription drugs in circulation worldwide may be counterfeit.
Today, this problem of illegal pharmaceutical products means that there are many risks to consumers, as drugs removed from the supply chain can be reintroduced to the market without thorough inspection processes - bypassing quality and safety controls.
Other problems related to adulterated or falsified medicines are associated with the possibility that they contain harmful dosages of different substances that are harmful to patients' health.
Therefore, the pharmaceutical industry and agencies responsible for drug distribution around the world are working on solutions that increase the safety, visibility and control of products in the supply chain to prevent counterfeiting.
In search of compliance? Learn what the DSCSA is
This acronym, having been present for some years in the work of those who deal with the movement of pharmaceutical products, identifies a legislative act, the Drug Supply Chain Security Act (DSCSA). Its purpose is to coordinate and inspect the creation of an interoperable electronic mechanism capable of identifying and tracking the distribution of medicines in the United States.
In the country, the Food and Drug Administration (FDA) is the agency responsible for managing and overseeing the approval and distribution of medicines.
DSCSA
The Drug Supply Chain Security Act (DSCSA) was passed in November 2013. It is intended to provide guidance to the FDA on new standards for drug distribution in the United States, making it easier to detect and remove potentially dangerous drugs from the pharmaceutical supply chain.
This law requires that there is traceability and reporting of product licensing from end to end within the supply chain, ensuring the safety of the end consumer.
Through an electronic communication model, the participants in the chain exchange important data with the responsible body (.XML standard), which makes the management of the movement and commercialization of medicines and prescription drugs in the country more efficient.
DSCSA: New Supply Chain Opportunities
The legislation and requirements on serialization and traceability of medicines ensure the safety of pharmaceutical products, combating theft, drug diverting and counterfeiting. These requirements also facilitate recalls and saleable returns within the supply chain, reducing costs and negative impacts on the business.
Furthermore, by having that visibility of information, the origin of the medicines can be ascertained. This directly impacts the safety of the end consumer and the reputation of the distributors participating in the chain.
Drug serialization and pharmaceutical packaging
The first step to efficient drug traceability is the implementation of serialization.
Serialization assigns a unique bar code, called a single code, to each medicine package, and it must be imprinted. The structure of this code is defined by specific US regulations, in line with GS1 standards.
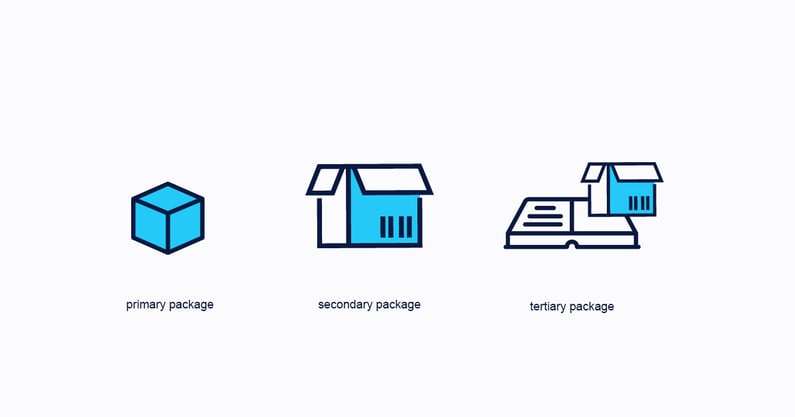
Primary Packaging
Is the packaging in contact with the pharmaceutical product.
Secondary packaging
This is the packaging that surrounds the primary packaging and is essential for serialization in accordance with the country's regulations.
Tertiary Packaging
Here you need to think of packages, boxes, and pallets. It is the most important packaging in drug serialization.
The two-dimensional GS1 DataMatrix code
Due to new regulations in product traceability, secondary packaging of drugs must now contain information regarding manufacturing.
The GS1 DataMatrix 2D code encodes more data than the traditional bar code, and more efficiently. It has therefore become the standard for the identification of pharmaceutical items.
What happened to the QR Code?
The QR Code is also a GS1 2D barcode format. It is similar to DataMatrix, but stores other types of data, such as the website address, and is widely used as a replacement for hyperlinks.
Because of this, QR Codes are still present in the experience of many consumers, who use them to get quick information about medicines and other products.
How to serialize drugs compliantly?
The use of the 2D DataMatrix code enables the data support that meets the US’s regulatory standards.
To effectively apply drug serialization, you need to incorporate the printed version of the code on the product packaging, containing the following information:
- GLN: the Global Location Number is the global location number that identifies localities and even legal bodies;
- GTIN: the Global Trade Item Number is a unique number for identifying products and services;
- Serial Number: a sequence of up to 20 characters unique to each GTIN;
- Lot Information
- 2D Bar Code.

Although there is a uniform standard and a global tracking number, serialization requirements vary between countries, with differences in small details that can be used for identification. It is therefore necessary to always be familiar with national laws and regulations.
GS1 Standards: data identification, capturing and sharing.
The standards established by GS1 help organizations in the process of serialization and traceability of medicines, distributing important information within the supply chain. Among this information is:
Identification
Involves the numbers and codes needed to identify drugs, services, assets, locations, and documents, for example. Among these codes we have the GTIN, the GLN, and the SSCC (Serial Shipping Container Code, used to identify logistical units).
Capturing
GS1 data carriers capture large amounts of data that solve regulatory needs in different territories such as lot number, expiration date, and serial number. The carriers include the 2D GS1 DataMatrix and EPC code.
Sharing
GS1 standards promote interoperability and support data exchange between trading partners in the supply chain, including EPCIS (Electronic Product Code Information Services, standard code for electronic sharing of event data between partners).
The importance of aggregation in serialization and product traceability
In drug traceability, every step of the products in the supply chain must be documented. Such movement is usually registered in codes printed on the tertiary packages, such as boxes or pallets.
By using these packages as support, the violation and potential damage to the products is avoided, since the secondary and primary packages are preserved. Another benefit is the agility in reading and collecting information about the pharmaceutical drugs.
However, it is important to note that the identification system in tertiary packaging, in order to bring positive benefits to the industry and or distributor, needs to be associated with the aggregation process. In other words, the individual drug data, normally printed on the primary packaging, needs to be aggregated into a single code that offers access to individualized data.
Beyond compliance: automating the supply chain
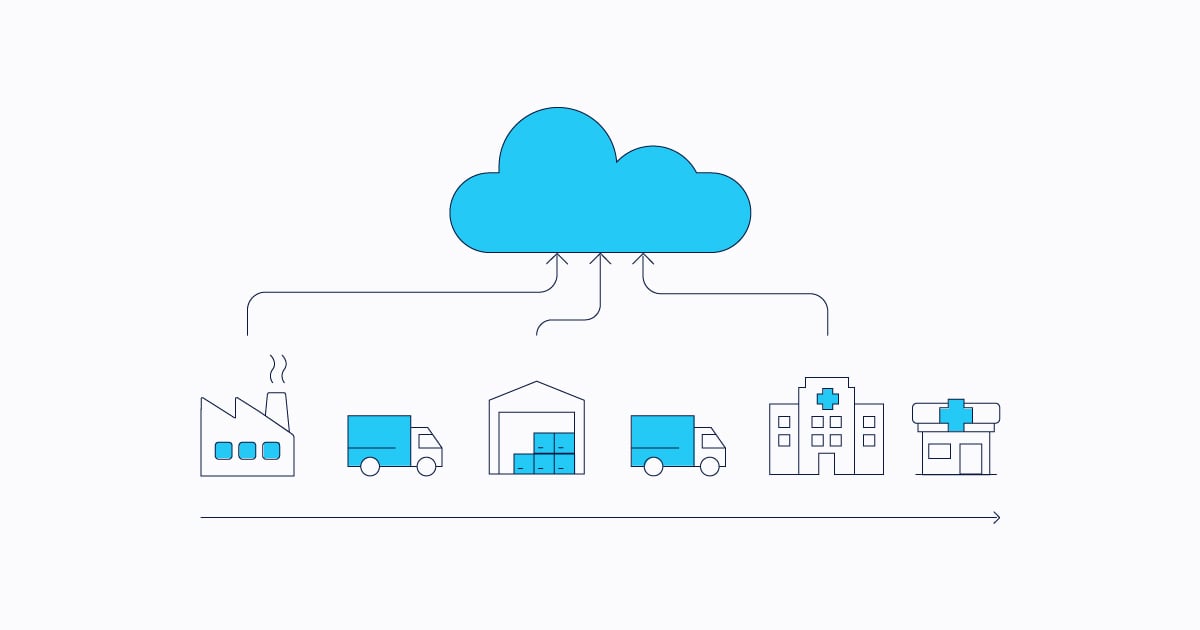
Automating the pharmaceutical supply chain with a complete serialization and traceability system is an opportunity to minimize challenges that may go beyond simply meeting compliance regulations.
With the Traceability Portal developed by TrackTraceRX, you automate your day-to-day processes, boost agility, improve performance, comply with regulatory legislation, and cure some common headaches:
- Simplify eventual product recall processes;
- Identify expired products before they enter the chain;
- Prevent drug shortages;
- Manage Master Data securely;
- Integrate its activities with those of your partners seamlessly.
With unlimited support and constant guidance, the TTRX team is always available to assist your company during the traceability implementation.
Request a demo now and discover the many benefits of an interoperable system.


COMMENTS